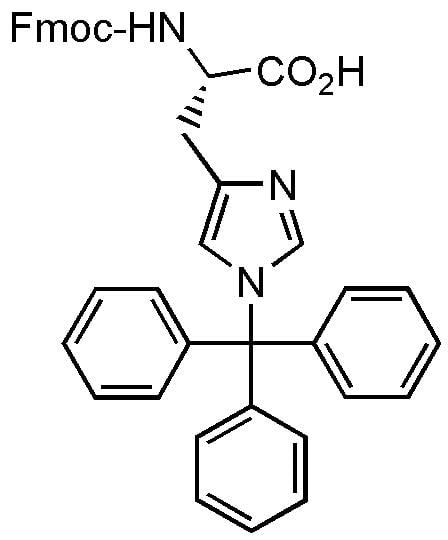
Fmoc-His(Trt)-OH – Building block in solid-phase peptide synthesis
Purchase Fmoc-His(Trt)-OH; N-Fmoc-N'-trityl-L-histidine; 109425-51-6
View Safety Data Sheet (SDS)
| 5g | $20.00 |
| 25g | $40.00 |
| 100g | $120.00 |
Fmoc-His(Trt)-OH can be used as a building block in solid-phase peptide synthesis (SPPS). Fmoc-His(Trt)-OH can undergo partial racemization during coupling easier than many other amino acids. Addition of HOBt or HOAt can minimize the racemization. See prices below.
| Catalog Number | FH2316 |
| InChI | InChI=1S/C40H33N3O4/c44-38(45)37(42-39(46)47-26-36-34-22-12-10-20-32(34)33-21-11-13-23-35(33)36)24-31-25-43(27-41-31)40(28-14-4-1-5-15-28,29-16-6-2-7-17-29)30-18-8-3-9-19-30/h1-23,25,27,36-37H,24,26H2,(H,42,46)(H,44,45)/t37-/m0/s1 |
| Stereoisomer Of | N-Fmoc-N'-trityl-D-histidine |
| DSSTox Substance ID | DTXSID80465310 |
| ECHA Substance Infocard ID | 100.108.322 |
| PubChem CID | 11422193 |
| Isomeric SMILES | C1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=CC=C3)N4C=C(N=C4)C[C@@H](C(=O)O)NC(=O)OCC5C6=CC=CC=C6C7=CC=CC=C57 |
| CAS Registry Number | 109425-51-6 |
| UniChem Compound ID | 22956595 |
| EC Number | 600-919-2 |
| Canonical SMILES | O=C(NC(Cc1cn(C(c2ccccc2)(c2ccccc2)c2ccccc2)cn1)C(=O)O)OCC1c2ccccc2-c2ccccc21 |
| Subclass Of | chemical compound |
| InChIKey | XXMYDXUIZKNHDT-QNGWXLTQSA-N |
| Instance Of | type of chemical entity |
| DSSTOX Compound Identifier | DTXCID20416129 |
| Mass | 619.2471065359999 |
| Chemical Formula | C₄₀H₃₃N₃O₄ |
Fmoc-His(Trt)-OH – Building block in solid-phase peptide synthesis
Fmoc-His(Trt)-OH – Building block in solid-phase peptide synthesis Related Compounds with Annotation
Fmoc-His(Trt)-OH – Building block in solid-phase peptide synthesis Depositor-Supplied Synonyms
Fmoc-His(Trt)-OH – Building block in solid-phase peptide synthesis R3D Conformer
Fmoc-His(Trt)-OH – Building block in solid-phase peptide synthesis GHS Classification
Buy Fmoc-His(Trt)-OH; N-Fmoc-N'-trityl-L-histidine; 109425-51-6 online.